CRM197
Many antigens are used as conjugates or conjugated vaccines because they have low immunogenicity, especially in infants, unless they are chemically linked to proteins. CRM197 is non-toxic at the genetic level and possesses a large complement of lysine that serve as advantages in conjugation. There are also studies showing that there is little carrier-induced immunosuppression inhibition compared to tetanus toxoid.
Using our proprietary technology, CRM197 was successfully produced in E. coli and purified with high yield. In our company, we keep searching its additional applications other than vaccine adjuvant of polysaccharide vaccines.
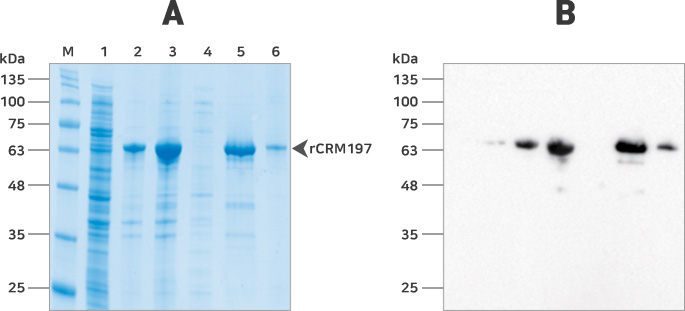
Fig. Purification of CRM197 solubilized from inclusion bodies. (A) SDS-PAGE analysis of each protein sample from each processing step. The dilution ratio of the insoluble fraction (lane 2) was the same as that of the soluble fraction (lane 1). (B) Western blot analysis of each protein sample fraction from each processing step. After transferring proteins into PVDF membrane, signal was detected signal using anti-DT antibody. M: molecular marker; lane 1, soluble fraction; lane 2, insoluble fraction; lane 3, proteins solubilized from inclusion bodies for loading Histrap column; lane 4, flow through fraction (His-trap column); lane 5, pooled elution fractions (Histrap column); lane 6, rCRM197 purified by size exclusion chromatography
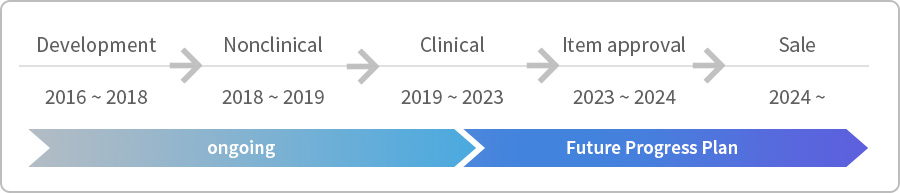
Development progress : CRM197